Abstract
Introduction: Approximately 10% of individuals with hematological malignancies are diagnosed with Multiple Myeloma (MM); in addition, the incidence and prevalence of the disease have progressively increased in recent decades. Despite the recent remarkable advances in treatment, autologous hematopoietic stem cell transplantation (ASCT) is still a fundamental part of the therapeutic management of this population. Particularly in developing countries, ASCT is an underused tool. Social and economic issues play an essential role in accessing the procedure. Refrigerated hematopoietic stem cells (R-HSC) can be an attractive alternative to the traditional use of cryopreserved hematopoietic stem cells (C-HSC) since cryopreservation demands highly trained human resources, high-cost supplies, and sophisticated equipment.
Aim: In order to compare the applicability, safety, and possible clinical and economic benefits of ASCT with different types of cell storage before infusion, we analyzed the medical records of patients diagnosed with MM who were transplanted using R-HSC or C-HSC.
Methods: The study was approved by the local Research Ethics Committee. We analyzed charts of patients diagnosed with MM and submitted to ASCT at Erasto Gaertner Hospital between January 2017 and July 2021.
R-HSC: Cells were stored immediately after leukapheresis, without any handling procedure, kept in a standard refrigerator at a temperature between 2-6oC, and infused within 48 hours after collection.
C-HSC: Cells were collected by leukapheresis and submitted to cryopreservation within 12 hours after the procedure. As a cryoprotectant, a solution with dimethyl sulfoxide (DMSO) at a final concentration of 5.5% was used. The product's temperature was reduced under a controlled cooling rate and stored in a freezer at -80oC.
All patients received a single intravenous dose of melphalan (200-100mg/m2) 24 hours before receiving the cells as conditioning regimen.
In the group of patients who received R-HSC, the cells were removed from the refrigerator and immediately infused at a rate of 10 ml/minute. However, in patients who received C-HSC, the cells were removed from the freezer, thawed in a water bath at 37oC, and infused intravenously at a rate of 10 ml/minute. All patients received G-CSF from D+5 until neutrophilic engraftment.
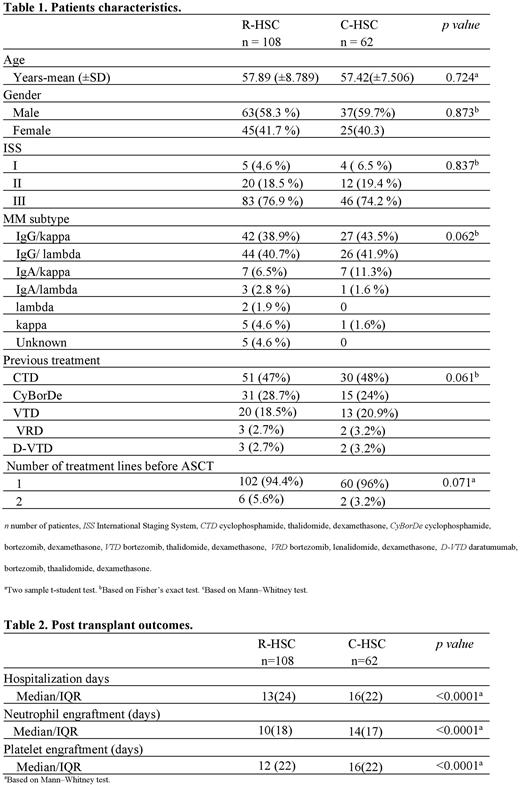
Results: We reviewed records of 170 patients. One hundred eight subjects received R-HSC and 62 C-HSC. Demographic characteristics, CD34+ cell dose received, melphalan dose, febrile neutropenia, and early mortality related to the procedure were similar between the two groups. There was a significant statistical difference in favor of the group utilizing R-HSC related to days until neutrophil engraftment (10 vs. 14 days p<0.0001), days until platelet engraftment (12 vs. 15 days p<0.0001), days of hospitalization (13 vs. 16 days p<0.0001) and infusion-related adverse reactions (10.1% vs. 33.8% p=0.003). There were no cases of graft failure in both groups. A reduction of 32.4% in the total cost per transplant was noticed in the R-HSC group.
Conclusions: We demonstrated in this analysis that the use of stem cells stored in a standard refrigerator, with a temperature between 2-6oC for up to 48 hours after the collection is a safe and efficient approach. It was capable of simplifying the ASCT, preventing infusion-related adverse reactions, assuring an early neutrophil and platelet engraftment, and reducing hospitalization days. This strategy should be considered especially in resource constrained settings to avoid unnecessary expenses, increase the number of transplant centers, and expand access to the procedure.
References:
1.Sarmiento M, Ramírez P, Parody R, et al.. Advantages of non-cryopreserved autologous hematopoietic stem cell transplantation against a cryopreserved strategy. Bone Marrow Transplantation. 2018;53(8):960-966.
Disclosures
Farias:Libbs Farmacêutica: Research Funding; Astrazeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; PPD: Research Funding; MSD: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal